The ONLY CGRP receptor antagonist nasal spray1,2
The ONLY CGRP receptor antagonist nasal spray1,2
ZAVZPRET is rapidly absorbed in the nasal cavity, directly entering the bloodstream, avoiding GI absorption and first-pass metabolism.1,3
Peak plasma concentration of zavegepant was observed at approximately 30 minutes after a single 10 mg dose of the nasal spray.1
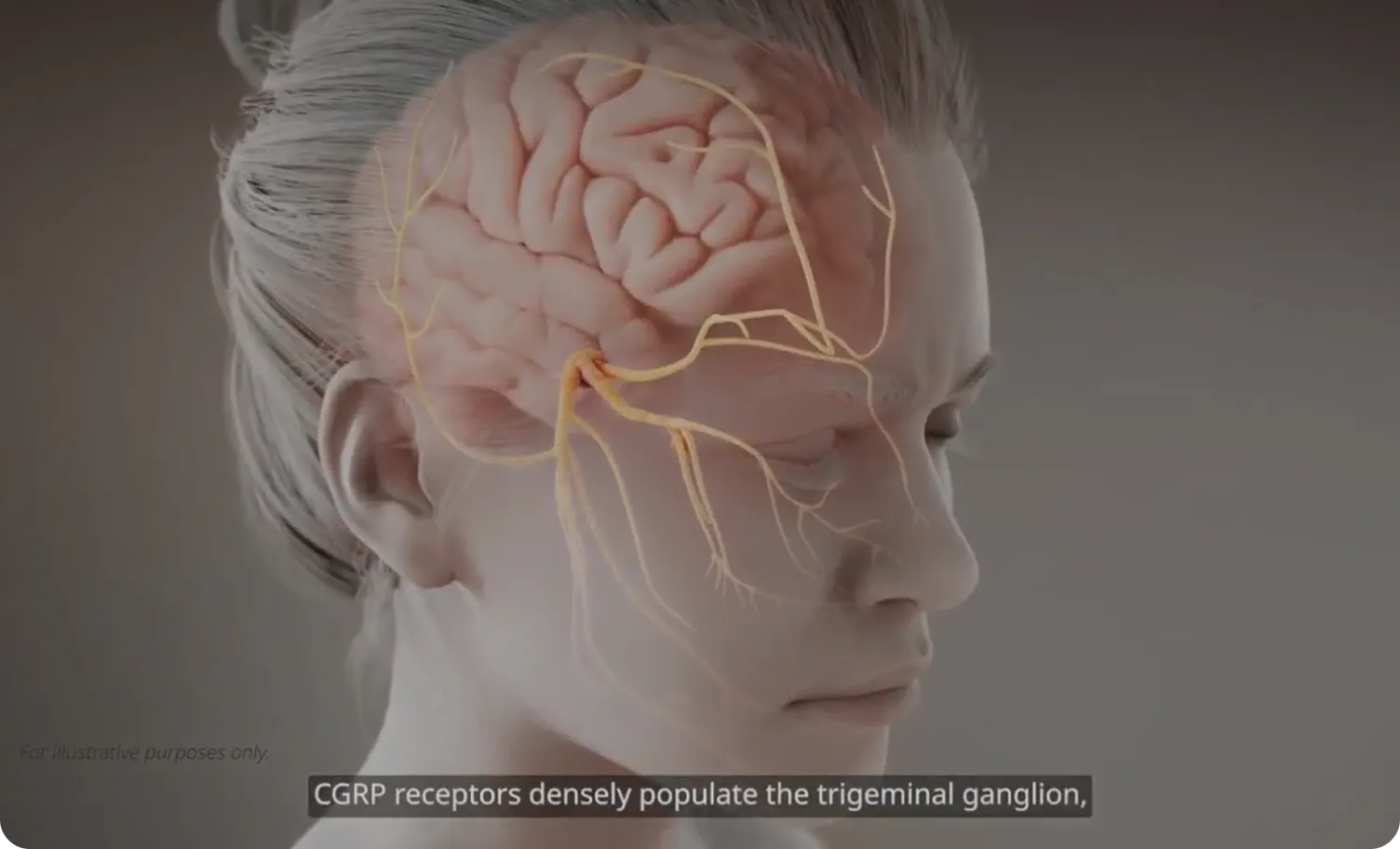
MOA=mechanism of action
MOA=mechanism of action
Ben is a teacher with migraine who often endures the pain while teaching.